Bifunctional nickel and copper electrocatalysts for CO2 reduction and the oxygen evolution reaction†
Abstract
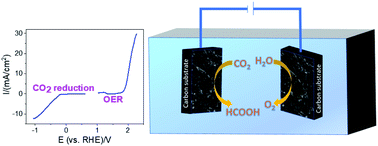
In this study, a bifunctional electrocatalyst for CO2 reduction and the O2 evolution reaction (OER) was constructed from the electrodeposition of cuprous oxide (Cu2O) and Ni on a carbon substrate. Different Ni thicknesses on Cu2O were achieved by varying the time of chronopotentiometric deposition of Ni. Electrochemical CO2 reduction was carried out at −0.89 V and −1.89 V vs. RHE, and it was found that formate and CO were the two major products. Cu2O modified with a Ni overlayer with a thickness of ∼700 nm resulted in the highest formate faradaic efficiency of 18%, and Cu2O resulted in highest CO faradaic efficiency of 7.9%. The enhanced faradaic efficiency for formate is attributed to the synergistic effect between Ni and Cu2O due to maximized amounts of exposed bimetallic sites that facilitate CO2 reduction. The electrocatalyst also produces ∼9 times more current density than previous studies using Ni–Cu2O electrocatalysts for the OER. The ability of the Ni–Cu2O thin films to catalyze both the OER and CO2 reduction allows them to be incorporated in the first demonstration of a two-electrode CO2 conversion device with a bifunctional catalyst. In this architecture, the device produces formate and CO with faradaic efficiencies of 16.0% and 19.7%, respectively.


 Please wait while we load your content...
Please wait while we load your content...