Unparalleled mitigation of membrane degradation in fuel cells via a counter-intuitive approach: suppression of H2O2 production at the hydrogen anode using a Ptskin–PtCo catalyst†
Abstract
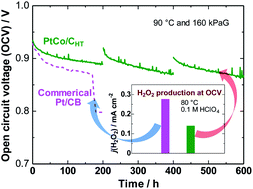
Hydroxyl radicals (˙OH) are responsible for much of the degradation of the proton exchange membrane (PEM) used in fuel cells. The conventional approach has been to use radical scavengers incorporated into the PEM, but performance is decreased. Here, we propose a counter-intuitive strategy in which the production of hydrogen peroxide, the precursor of ˙OH, is suppressed at the hydrogen anode, where oxygen diffusing from the cathode is reduced by adsorbed hydrogen atoms. This is accomplished via the use of a Pt skin-covered PtCo alloy anode catalyst, on which H is weakly adsorbed, as indicated by theoretical calculations. In particular, the H2O2 production on the hydrogen anode at a practical temperature of 80 °C was, for the first time, evaluated by the application of the channel flow double electrode (CFDE) technique. A remarkably longer lifetime of a PEM with the PtCo/C anode, in comparison with that for a commercial Pt/C anode, has been demonstrated in an accelerated stress test of a single cell (open circuit under pressurized gases).


 Please wait while we load your content...
Please wait while we load your content...