Iron single-atom catalyst anchored on nitrogen-rich MOF-derived carbon nanocage to accelerate polysulfide redox conversion for lithium sulfur batteries†
Abstract
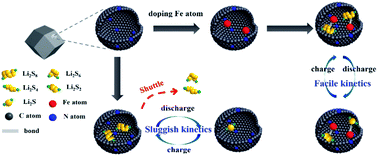
Lithium sulfur batteries (LSBs) have been strongly considered the most promising candidate for next-generation energy storage devices owing to their higher energy density. However, the dissolution of lithium polysulfides (LiPSs) and sluggish conversion kinetics during charge/discharge cycling impede the LSBs. Herein, we employed the nanocage-like nitrogen-rich metal organic framework (MOF)-derived carbon decorated with an iron single-atom (FeSA–CN) catalyst to trigger the surface-mediated reaction of LiPSs. The synergistic effect from the iron single atom (FeSA) and nitrogen-rich porous carbon significantly boost the reaction kinetics and utilization of the sulfur species. The FeSA–CN/S electrode delivered a specific capacity of 1123 mA h g−1 at 0.2C, and exhibited an excellent rate performance of 605 mA h g−1 at 4.0C with an ultralow capacity fading rate of 0.06% per cycle for 500 cycles. This work provides an effective strategy to couple the function of a nanoporous material host and single-atom catalyst for lithium sulfur batteries.
- This article is part of the themed collection: Journal of Materials Chemistry A Lunar New Year collection 2021


 Please wait while we load your content...
Please wait while we load your content...