Facile aqueous synthesis of high performance Na2FeM(SO4)3 (M = Fe, Mn, Ni) alluaudites for low cost Na-ion batteries†
Abstract
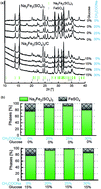
A new method of synthesis of stoichiometric Na2FeM(SO4)3 (M = Fe, Mn, Ni) materials is developed. The proposed unique methodology is based on a facile processing of dissolution of Na2SO4 and transition metal (Fe, Mn, and Ni) sulfates in an aqueous solution, solvent evaporation, and annealing. This novel method is characterized by a very low degree of complexity but still ensures the highest purity, resulting in the high purity of Mn and Ni substituted alluaudite compounds. Synthesized materials possess nanometric size grains with uniform composition distribution. Na2FeM(SO4)3 exhibits great transport properties with low charge transport activation energy related to sodium ion diffusion. Electrochemical characterization of the processed Na2Fe2(SO4)3 compound in both half-cell and full cell formats demonstrates the highest power density among all polyanion Na cathodes.


 Please wait while we load your content...
Please wait while we load your content...