Interlayer separation in hydrogen titanates enables electrochemical proton intercalation†
Abstract
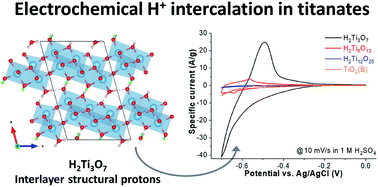
Electrochemical proton intercalation into titanium oxides is typically limited to the near-surface region, necessitating the use of nanostructured high surface area materials to obtain high capacities. Here, we investigate the role of materials structure to extend intercalation beyond the near-surface region. Employing a series of hydrogen titanates (HTOs), we find that electrochemical protonation capacity significantly increases with interlayer structural protonation. The maximum capacity of ∼80 mA h g−1 is achieved with H2Ti3O7, which also undergoes reversible structural and optical changes during the de/intercalation process. Using quasi-elastic neutron scattering, we show that structural protons are highly confined in the HTO interlayer with only localized, but not translational, dynamics. Electrochemical protonation leads to a contraction of the H2Ti3O7 interlayer without causing significant strain in the two-dimensional titanate layers. Density functional theory calculations indicate more favorable adsorption energy for intercalated protons in H2Ti3O7 as compared to HTOs with fewer structural protons. We hypothesize that interlayer structural protons are the structural feature responsible for the high electrochemical protonation capacity in H2Ti3O7 because they effectively decrease the interconnections between the titanate layers. This enables facile compensation of the electrochemically introduced strain via one-dimensional interlayer contraction. These results demonstrate the special structural requirements for bulk proton intercalation in titanium oxides, and offer a new materials design strategy for high energy density aqueous energy storage.


 Please wait while we load your content...
Please wait while we load your content...