Anion-exchange phase control of manganese sulfide for oxygen evolution reaction†
Abstract
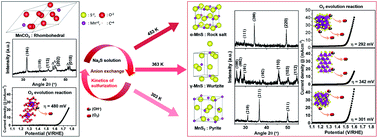
Manganese sulfide usually exists as MnS2 (cubic), β-MnS (zinc blende), γ-MnS (wurtzite), and α-MnS (rock salt) polymorphs. In this study, polymorphic thin films of MnS2, γ-MnS, and α-MnS were prepared from manganese carbonate through anion exchange and controlled reaction kinetics via a hydrothermal method. At the same mass ratio (1 : 1) of manganese carbonate to the S precursor, the hydrothermal anion-exchange kinetics were used to determine the phases (MnS2, γ-MnS, and α-MnS) of manganese sulfide. The α-MnS phase was obtained at a high S precursor content, specifically at a mass ratio of 1 : 5, even at 363 K. The different prepared phases (MnS2, γ-MnS, and α-MnS) exhibited superior O2 evolution reaction activities (overpotential: 301, 342, and 292 mV, respectively) compared with pristine manganese carbonate (overpotential: 480 mV) in a 1 M KOH electrolyte to drive a current density of 10 mA cm−2. Among all the polymorphs, α-MnS exhibited the lowest overpotential because it had the lowest charge-transfer resistance (8.9 Ω cm−2), edge sharing MnS6 octahedra with more active sites and highest electrochemical active surface area (360 cm−2). This study provides a new approach to control the phase of transition-metal chalcogenides for improved electrochemical reactions.


 Please wait while we load your content...
Please wait while we load your content...