Mechanism of CO production around oxygen vacancy of LaMnO3: an efficient and rapid evaluation of the doping effect on the kinetics and thermodynamic driving force of CO2-splitting
Abstract
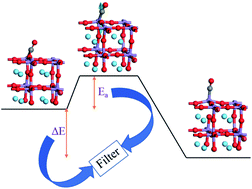
Lanthanum–manganese perovskites have been shown to be promising oxygen carriers for the solar-driven thermochemical production of CO due to their low reduction temperature and considerable CO production. However, to increase their energy conversion efficiency, it is also important to overcome the CO2-splitting thermodynamic constraints and reduce the time for the complete re-oxidation of perovskites. Thus, herein, the mechanism of CO production around the oxygen vacancy of LaMnO3 has been elucidated for the first time. One transition state with an activation energy (Ea) of 19 kJ mol−1 was explored and confirmed by the quantity and direction of the imaginary frequency. Based on the analysis of Ea, the evaluated ratio of the CO2-splitting time for La1−xSrxMnO3 (x = 0.1, 0.2 and 0.3) is 1 : 5.2 : 32.8, which is similar to the experimental ratio of 1 : 4.5 : 29.3. Furthermore, the kinetic characteristics of La0.625Sr0.375Mn0.5B0.5O3 and La0.625Ca0.375Mn0.5B0.5O3 (B = Al, Ga, Cr and Fe) were predicted, which showed good consistency with experiments. In addition, we found a strong negative correlation between the thermodynamic driving force and the energy difference (ΔE) between the CO2 and CO adsorption configurations. Thus, all the results indicate the reliability of Ea and ΔE in the prediction and evaluation of the CO2-splitting activity. Finally, La0.625Sr0.375Mn0.5Cr0.5O3 and La0.625Ca0.375Mn0.5Cr0.5O3 are suggested as potential STC materials with a fast CO2-splitting rate and high solar-to-fuel efficiency.


 Please wait while we load your content...
Please wait while we load your content...