Temperature tunability in Sr1−xCaxFeO3−δ for reversible oxygen storage: a computational and experimental study†
Abstract
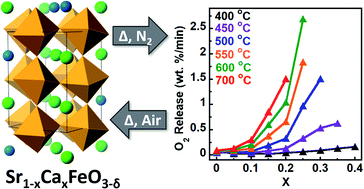
Reversible oxygen absorption/desorption in earth-abundant materials is fundamental towards many advanced technologies that require a pure oxygen stream including oxy-combustion via chemical looping and oxygen-blown coal gasification. The Sr1−xCaxFeO3 system is one of many perovskite oxides that have drawn attention given the large quantities of oxygen it can produce during mild cycling conditions. In this work, we employ hybrid functional based DFT calculations to establish the oxygen vacancy formation energy, ΔEf,vac, as an effective parameter for describing the Sr1−xCaxFeO3 system. We validate these calculations experimentally using XPS, O2-TPD, TGA, and synchrotron pXRD. In all cases, we find that increasing calcium content lowers both the oxygen desorption temperature and preferred operating temperature for the system. Importantly, we are able to experimentally show the structural and functional cyclability of these materials, achieving rapidly accessible maximum oxygen storage capacities of 1.51–2.41 wt% for temperatures of 400–700 °C.


 Please wait while we load your content...
Please wait while we load your content...