Supramolecular hydrogels from unprotected dipeptides: a comparative study on stereoisomers and structural isomers†
Abstract
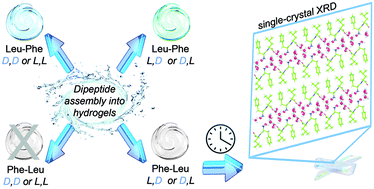
Amino acid stereoconfiguration has been shown to play a key role in the self-assembly of unprotected tripeptides into hydrogels under physiological conditions. Dramatic changes were noted for hydrophobic sequences based on the diphenylalanine motif from the formation of amorphous aggregates in the case of homochiral peptides to nanostructured and stable hydrogels in the case of heterochiral stereoisomers. Herein, we report that by further shortening the sequence to a dipeptide, the overall differences between isomers are less marked, with both homo- and hetero-chiral dipeptides forming gels, although with different stability over time. The soft materials are studied by a number of spectroscopic and microcopic techniques, and single-crystal X-ray diffraction to unveil the supramolecular interactions of these hydrogel building blocks.
- This article is part of the themed collections: Soft Matter Lectureship Winners and Peptide Soft Materials


 Please wait while we load your content...
Please wait while we load your content...