Micellar structure and transformations in sodium alkylbenzenesulfonate (NaLAS) aqueous solutions: effects of concentration, temperature, and salt†
Abstract
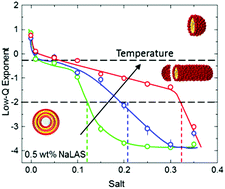
We investigate the shape, dimensions, and transformation pathways of micelles of linear sodium alkylbenzenesulfonate (NaLAS), a common anionic surfactant, in aqueous solution. Employing Small Angle Neutron Scattering (SANS) and surface tensiometry, we quantify the effects of surfactant concentration (0.6–15 wt%), temperature (5–40 °C) and added salt (≤0.35 M Na2SO4). Spherical micelles form at low NaLAS (≤2.6 wt%) concentration in water, and become elongated with increasing concentration and decreasing temperature. Addition of salt reduces the critical micelle concentration (CMC) and thus promotes the formation of micelles. At fixed NaLAS concentration, salt addition causes spherical micelles to grow into cylindrical micelles, and then multilamellar vesicles (MLVs), which we examine by SANS and cryo-TEM. Above a threshold salt concentration, the MLVs reach diameters of 100 s of nm to few μm, eventually causing precipitation. While the salt concentrations associated with the micelle-to-cylinder transformation increase only slightly with temperature, those required for the cylinder-to-MLV transformation exhibit a pronounced, linear temperature dependence, which we examine in detail. Our study establishes a solution structure map for this model anionic surfactant in water, quantifying the combined roles of concentration, temperature and salt, at practically relevant conditions.


 Please wait while we load your content...
Please wait while we load your content...