Ubiquity of complex coacervation of DNA and proteins in aqueous solution†
Abstract
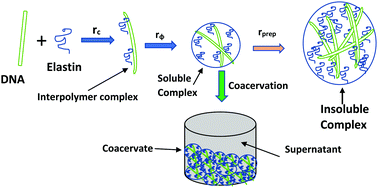
We report complex coacervation between a primarily hydrophobic protein, elastin, and a strong polyanion DNA (2 kbp) in aqueous and salty solutions at room temperature, 25 °C. The associative interaction at fixed elastin and varying DNA concentration, thereby maintaining a mixing ratio of r = [DNA] : [elastin] = 0.0027 to 0.093, was probed. What distinguishes this study from protein–DNA coacervation reported earlier is that the protein used here was mostly a hydrophobic polyampholyte with low linear charge density, and its complementary polyelectrolyte, DNA, concentration was chosen to be extremely small (1–35 ppm). The interaction profile was found to be strongly hierarchical in the mixing ratio, defined by three distinct regions: (i) Region I (r < 0.02) was defined as the onset of primary binding leading to condensation of DNA; (ii) Region II (0.02 < r < 0.08) indicated secondary binding which led to the formation of fully charge neutralized complexes signaling the onset of coacervation; and (iii) Region III (0.08 < r < 0.12) revealed growth of insoluble complexes of large size facilitating liquid–solid phase separation. The degree of complex coacervation was suppressed in the presence of a monovalent salt implying that screened Coulomb interactions governed the binding. Small angle neutron scattering data attributed an amorphous structure to the coacervates. The elastin–DNA system belongs to a rare class of interacting biopolymers where very weak electrostatic interactions may drive coacervation, thereby implying that coacervation between DNA and proteins may be ubiquitous.


 Please wait while we load your content...
Please wait while we load your content...