Hydrogen bonding and charge transport in a protic polymerized ionic liquid†
Abstract
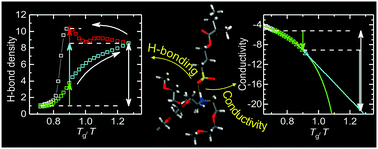
Hydrogen bonding and charge transport in the protic polymerized ionic liquid poly[tris(2-(2-methoxyethoxy)ethyl)ammoniumacryloxypropyl sulfonate] (PAAPS) are studied by combining Fourier transform infrared (FTIR) and broadband dielectric spectroscopy (BDS) in a wide temperature range from 170 to 300 K. While the former enables to determine precisely the formation of hydrogen bonds and other moiety-specific quantized vibrational states, the latter allows for recording the complex conductivity in a spectral range from 10−2 to 10+9 Hz. A pronounced thermal hysteresis is observed for the H-bond network formation in distinct contrast to the reversibility of the effective conductivity measured by BDS. On the basis of this finding and the fact that the conductivity changes with temperature by orders of magnitude, whereas the integrated absorbance of the N–H stretching vibration (being proportional to the number density of protons in the hydrogen bond network) changes only by a factor of 4, it is concluded that charge transport takes place predominantly due to hopping conduction assisted by glassy dynamics (dynamic glass transition assisted hopping) and is not significantly affected by the establishment of H-bonds.


 Please wait while we load your content...
Please wait while we load your content...