Effect of the C-terminal amino acid of the peptide on the structure and mechanical properties of alginate–peptide hydrogels across length-scales†
Abstract
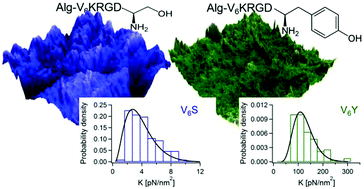
Alginate is a natural anionic polysaccharide that exhibits excellent biocompatibility and biodegradability. Alginate hydrogels have many different applications in the field of regenerative medicine especially when peptides are conjugated to the alginate backbone. Here, we systematically investigate the effect of six arginine–glycine–aspartic acid (RGD)-containing peptides, G6KRGDY/S, A6KRGDY/S and V6KRGDY/S, on the macroscopic and microscopic physical properties and spatial organization of alginate–peptides hydrogels. Using rheology, small angle X-ray scattering and nanoindentation measurements we show a strong correlation between the macroscopic-bulk properties and the microscopic-local properties of the alginate–peptide hydrogels. Furthermore, our results indicate that the identity of the amino acid at the C-terminal of the peptide plays a major role in determining the structure and mechanical properties of the hydrogel across length-scales, where the presence of tyrosine (Y) terminated peptides introduce more junction-zones and consequently larger stiffness than those terminated with serine (S).


 Please wait while we load your content...
Please wait while we load your content...