How do chemical patterns affect equilibrium droplet shapes?†
Abstract
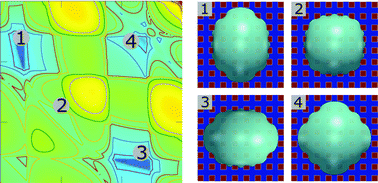
By utilizing a proposed analytical model in combination with the phase-field method, we present a comprehensive study on the effect of chemical patterns on equilibrium droplet morphologies. Here, three influencing factors, the droplet sizes, contact angles, and the ratios of the hydrophilic area to the hydrophobic area, are contemplated. In the analytical model, chemical heterogeneities are described by different non-linear functions. By tuning these functions and the related parameters, the analytical model is capable of calculating the energy landscapes of the system. The chemically patterned surfaces display complex energy landscapes with chemical-heterogeneity-induced local minima, which correspond to the equilibrium morphologies of the droplets. Phase-field (PF) simulations are accordingly conducted and compared with the predicted equilibrium morphologies. In addition, we propose a modified Cassie–Baxter (CB) model to delineate the equilibrium droplet shapes. In contrast to the classic CB model, our extension is not only restricted to the shape with a spherical cap. Both the energy landscape method and the modified CB model are demonstrated to have a good agreement with the PF simulations.


 Please wait while we load your content...
Please wait while we load your content...