Boronic acid and diol-containing polymers: how to choose the correct couple to form “strong” hydrogels at physiological pH†
Abstract
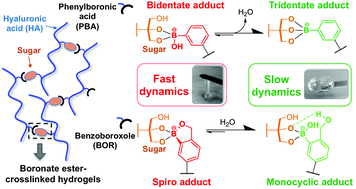
Dynamic covalent hydrogels crosslinked by boronate ester bonds are promising materials for biomedical applications. However, little is known about the impact of the crosslink structure on the mechanical behaviour of the resulting network. Herein, we provide a mechanistic study on boronate ester crosslinking upon mixing hyaluronic acid (HA) backbones modified, on the one hand, with two different arylboronic acids, and on the other hand, with three different saccharide units. Combining rheology, NMR and computational analysis, we demonstrate that carefully selecting the arylboronic-polyol couple allows for tuning the thermodynamics and molecular exchange kinetics of the boronate ester bond, thereby controlling the rheological properties of the gel. In particular, we report the formation of “strong” gels (i.e. featuring slow relaxation dynamics) through the formation of original complex structures (tridentate or bidentate complexes). These findings offer new prospects for the rational design of hydrogel scaffolds with tailored mechanical response.


 Please wait while we load your content...
Please wait while we load your content...