How an organogelator can gelate water: gelation transfer from oil to water induced by a nanoemulsion†
Abstract
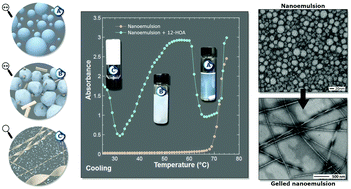
A hydrogel can be formed by an organogelator in the presence of a nanoemulsion. It is expected that this is due to a gelation transfer from oil to water. The system started with an oil-in-water nanoemulsion prepared according to a phase inversion temperature (PIT) process. Into this nanoemulsion consisting of Kolliphor® RH40 and Brij® L4 as surfactants, and Miglyol® 812 as oil and water, we introduced the organogelator 12-hydroxyoctadecanoic acid (12-HOA) in the oil phase. After cooling at room temperature, a slow reversible gelation of the water phase occurred with persistence of the nanoemulsion. This thermally reversible system was investigated using various techniques (rheology, turbidimetry, optical and electron microscopies, scattering techniques). Successive stages appeared during the cooling process after the nanoemulsion formation, corresponding to the migration and self-assembly of the organogelator from the oil nanodroplets to the water phase. According to our measurements and the known self-assembly of 12-HOA, a mechanism explaining the formation of the gelled nanoemulsion is proposed.


 Please wait while we load your content...
Please wait while we load your content...