Nanoconfined gelation of polyacrylonitrile, poly(vinyl alcohol), and isotactic polypropylene probed by calorimetry
Abstract
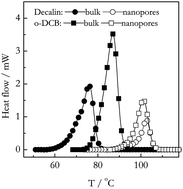
Differential scanning calorimetry is used to obtain insights into the kinetics and thermodynamics of nanoconfined gelation. Gels of polyacrylonitrile in propylene carbonate, poly(vinyl alcohol) in ethylene glycol, and isotactic polypropylene in o-dichlorobenzene and decalin are studied in silica nanopores. Two major effects are observed for nanoconfined gels: a decrease in the heat of gelation and an increase in the gelation temperature. The smaller heat indicates that nanoconfinement of polymer chains results in the formation of fewer ordered crosslinks. The increased gelation temperature suggests acceleration of the gelation kinetics. The kinetics has been treated by an advanced isoconversional method and interpreted in terms of the Fisher–Turnbull model. It is found that acceleration of gelation in nanopores is associated with a decrease in the free energy barrier to nucleation, as one would expect for a change in the process mechanism from homogenous to heterogenous nucleation.


 Please wait while we load your content...
Please wait while we load your content...