An Li+-enriched Co2+-induced metallogel: a study on thixotropic rheological behaviour and conductance†
Abstract
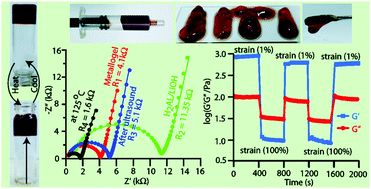
An alkali base and counterion-selective red metallogel (1% w/v) has been synthesized by mixing the adipic acid-derived ligand H2AL with LiOH, followed by the addition of 1 equivalent of Co(OAc)2 in DMF. The addition of Co(OAc)2 not only resulted in the formation of a 2 : 2 (M : L) complex, but also led to the consecutive steps of aggregation, fiber creation, entrapment of the solvent and eventually gelation. The metallogel formation and the mechanism behind gelation have been well characterized and established using various instrumental techniques such as FTIR spectroscopy, UV-vis spectroscopy, FE-SEM, TEM, PXRD, ESI-mass spectrometry, Job's plot and rheology analysis. Nyquist plots suggested a large decrease in the resistance value from 11.3 kΩ to 4.2 kΩ for the solution obtained from the ligand deprotonated by LiOH (AL2−) and Co(OAc)2 containing the metallogel. The Nyquist plot and resistance of the metallogel have also been studied under the influence of temperature and ultrasound stimuli. The extensive rheological measurements provide information about the strength of the gel network and the highly reversible nature and thixotropic behaviour of the metallogel.


 Please wait while we load your content...
Please wait while we load your content...