Oxidative instability of boronic acid-installed polycarbonate nanoparticles†
Abstract
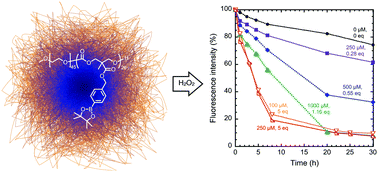
Oxidative stress, caused by the overproduction of reactive oxygen species (ROS), is often observed in degenerative and/or metabolic diseases, tumors, and inflamed tissues. Boronic acids are emerging as a unique class of responsive biomaterials targeting ROS because of their reactivity toward H2O2. Herein, we examine the oxidative reactivity of nanoparticles from a boronic acid-installed polycarbonate. The extent of oxidation under different concentrations of H2O2 was tracked by the change in fluorescence intensity of an encapsulated solvatochromic reporter dye, demonstrating their sensitivity to biologically-relevant concentrations of hydrogen peroxide. Oxidation-triggered particle destabilization, however, was shown to be highly dependent on the concentration of the final oxidized polymer product, and was only achieved if it fell below polymer critical micelle concentration. Our results indicate that these nanocarriers serve as an excellent dual pH/H2O2 responsive vehicle for drug delivery.


 Please wait while we load your content...
Please wait while we load your content...