ENTH domain-dependent membrane remodelling
Abstract
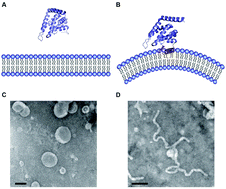
Cellular membranes are anything but flat structures. They display a wide variety of complex and beautiful shapes, most of which have evolved for a particular physiological reason and are adapted to accommodate certain cellular demands. In membrane trafficking events, the dynamic remodelling of cellular membranes is apparent. In clathrin-mediated endocytosis for example, the plasma membrane undergoes heavy deformation to generate and internalize a highly curved clathrin-coated vesicle. This process has become a model system to study proteins with the ability to sense and induce membrane curvature and over the last two decades numerous membrane remodelling molecules and molecular mechanisms have been identified in this process. In this review, we discuss the interaction of epsin1 ENTH domain with membranes, which is one of the best-studied examples of a peripheral and transiently membrane bending protein important for clathrin-mediated endocytosis.
- This article is part of the themed collection: Remodelling of Biomembranes


 Please wait while we load your content...
Please wait while we load your content...