Multivalent counterions accumulate in star-like polyelectrolytes and collapse the polymer in spite of increasing its ionization†
Abstract
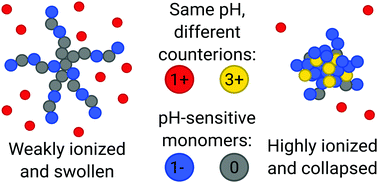
We used computer simulations to explore the dissociative and conformational behaviour of branched weak polyelectrolytes with multivalent counterions. We compared simulated titration curves and chain sizes in the presence of added salt of various valencies, keeping the total charge of salt constant. We showed that multivalent counterions enhance ionization of the weak polyelectrolytes, in spite of collapsing of the chains. We provided evidence that such an effect is absent in systems with only monovalent counterions at the same ionic strength, and thus cannot be attributed to electrostatic screening. We attributed it to strong ion–ion correlations that we quantified by comparing potentials of mean force with the mean electrostatic potentials. Finally, we used the partition coefficient to quantify the ability of star-like polyelectrolytes to capture multivalent ions, that is important for water-treatment applications. Our work provides fundamental understanding of the mechanism of polyelectrolyte collapse and ionization response upon addition of multivalent ions.


 Please wait while we load your content...
Please wait while we load your content...