Surface tension in liquids containing antagonistic ions
Abstract
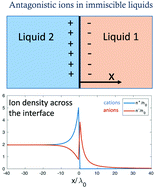
We use a modified Poisson–Boltzmann formalism to examine immiscible electrolytes containing dissolved antagonistic ions with arbitrary preferential solubilities. We solve the nonlinear equation and obtain an analytical expression for the potential profile, ion densities, and surface tension. Our model takes into account the dependence of the Debye lengths in the two liquids on the preferential solvation. In the limit of point-like ions, the surface tension scales with the average ion density n0 as the classical n01/2 power law. At larger densities ion crowding at the interface leads to a crossover to smaller exponents. The dependence of the surface tension on the Gibbs transfer energy is non-monotonic and exhibits a maximum or a minimum. We further look at a liquid bilayer confined in a parallel-plate capacitor and subject to an external potential. The ion distribution depends on whether the external potential has the same or opposite sign as the Donnan potential. Lastly, we calculate the surface tension of a liquid–liquid interface that has a sinusoidal height modulation and find that surface modulations increase the energy.


 Please wait while we load your content...
Please wait while we load your content...