Collective and contractile filament motions in the myosin motility assay†
Abstract
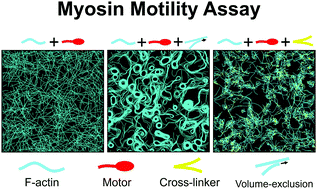
Cells require mechanical forces for their physiological functions. The forces are generated mainly from molecular interactions between actin filaments, cross-linking proteins, and myosin motors in the actin cytoskeleton. To better understand the molecular interactions, many studies employed myosin motility assays with actin filaments propelled by myosin heads fixed on a surface. Various interesting behaviors of actin filaments have been observed in the motility assay experiments. Despite the popularity of the motility assays, there were only a few computational models designed for simulating the motility assay systems. Most of the previous models have limitations which precluded full understanding of molecular origins for behaviors of actin filaments. In this study, we used an agent-based computational model based on Brownian dynamics for simulating the motility assay system. Our model rigorously describes the mechanics, dynamics, and interactions of actin filaments, cross-linking proteins, and molecular motors. Using the model, we first investigated how properties of actin filaments and motors affect gliding motions of actin filaments without volume-exclusion effects as a base study. We found that actin filaments can continuously glide at relative fast speed only when they are sufficiently longer than the average spacing between neighboring motors and that the gliding speed of F-actins shows a biphasic dependence on processivity of motors. Then, we showed that volume-exclusion effects between actin filaments can induce diverse collective movements and alignment of actin filaments, thus creating thick bundles and ring-like structures in the absence of cross-linking proteins. Lastly, we demonstrated that cross-linking proteins can lead to distinct contractile behaviors of actin networks depending on the density and kinetics of the cross-linking proteins. Results from our study show the ability of our model to simulate the motility assay system under various conditions and provide insights into understanding of different behaviors of actin filaments.
- This article is part of the themed collection: Active Matter


 Please wait while we load your content...
Please wait while we load your content...