Evidence for water ridges at oil–water interfaces: implications for ion transport†
Abstract
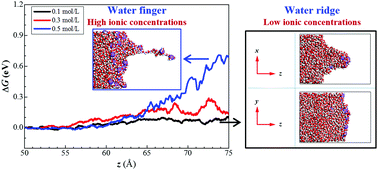
Understanding ion transport across interfaces is of fundamental importance in many processes such as liquid–liquid extraction, phase transfer catalysis, enhanced oil recovery and emulsion stabilisation. However, the factors that control ion transport across interfaces are poorly known due to a lack of knowledge of structural changes at interfaces. We studied here the effects of ionic concentration and external force on the transport of ions across the decane–water interface using classical molecular dynamics simulations. The results show that the evolution of interfacial structures during ion transfer across the interface is controlled by hydrogen bonding and ionic interactions at the interface. We also identified a new mode of ion transfer across the interface at low ionic concentrations, involving a ‘water ridge’, rather that the classical ‘water finger’. In the water ridge mode, hydrogen bonds are not broken due to low ion levels, and the water ridge induces gradual interface deformation. Whereas, at high ionic concentrations, hydrogen bonds are broken by the strong ion electrostatic repulsion, thus inducing the formation of a water finger. We also found that the variation of the Gibbs free energy during ion transfer is directly relevant to the ionic concentration. The water ridge at low ionic concentrations, which displaces more water molecules towards the decane phase, induces less free energy variation than the water finger at high ionic concentrations.


 Please wait while we load your content...
Please wait while we load your content...