Spatiotemporal pattern formation in E. coli biofilms explained by a simple physical energy balance†
Abstract
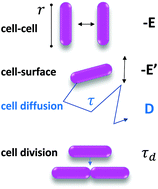
While the biofilm growth mode conveys notable thriving advantages to bacterial populations, the mechanisms of biofilm formation are still strongly debated. Here, we investigate the remarkable spontaneous formation of regular spatial patterns during the growth of an Escherichia coli biofilm. These patterns reported here appear with non-motile bacteria, which excludes both chemotactic origins and other motility-based ones. We demonstrate that a minimal physical model based on phase separation describes them well. To confirm the predictive capacity of our model, we tune the cell–cell and cell–surface interactions using cells expressing different surface appendages. We further explain how F pilus-bearing cells enroll their wild type kindred, poorly piliated, into their typical pattern when mixed together. This work supports the hypothesis that purely physicochemical processes, such as the interplay of cell–cell and cell–surface interactions, can drive the emergence of a highly organized spatial structure that is potentially decisive for community fate and for biological functions.


 Please wait while we load your content...
Please wait while we load your content...