Theoretical investigations of electrochemical CO2 reduction by transition metals anchored on CNTs†
Abstract
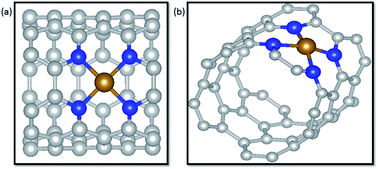
Transition metals supported on nitrogen doped carbon materials are a class of promising electrochemical catalysts toward electrochemical CO2 reduction reactions (CO2RR) that have exhibited excellent catalytic performance. Herein, M–N4 (M = Fe, Co and Ni) coordination structures embedded in carbon nanotubes (CNTs) were constructed to explore detailed mechanisms as electrocatalysts for CO2RR via density functional theory (DFT) calculations. Nitrogen atoms of coordination structures rather than only single transition metal atoms were demonstrated to be effective active sites toward CO2RR. For two possible pathways of the first step, forming *COOH or *OCHO, according to the catalytic activity of nitrogen atoms to the H atom, *COOH generation was facilitated in terms of kinetics over *OCHO in three systems. Meantime, it is suggested that the products of electrochemical CO2RR on the three catalysts are heavily dependent on the interaction of CO and the catalysts. Ni–N4/CNT exhibits a considerable selectivity to CO due to the weak interaction of CO and the substrates with a limiting potential of 1.79 V. On the contrary, CO prefers to remain on Fe–N4/CNT due to the strong binding energy of CO adsorbed on Fe–N4/CNT. Then the CO of the Fe–N4/CNT system undergoes further hydrogenation to produce CH3OH and CH4 at the same limiting potential of 0.68 V, indicating that there is no distinct selectivity in forming CH3OH and CH4. Nevertheless, the limiting potentials of CO, CH3OH and CH4 on Co–N4/CNT are totally different, 0.43 V, 0.56 V and 0.87 V, respectively. In particular, CO2RR to CO on Co–N4/CNT has the lowest potential limit. Thus, Co–N4/CNT has a considerable potential as the catalyst for electrochemical CO2RR. In addition, the catalysts of Ni single atoms, unsaturated coordination with nitrogen atoms, edge-anchored on CNTs were investigated for their activity for the CO2RR. Our comprehensive understanding of M–N4/CNT materials may be instructive and meaningful to design advanced catalysts from nonprecious metals for electrochemical CO2RR.


 Please wait while we load your content...
Please wait while we load your content...