CO2 methanation over Ni–Al and Co–Al LDH-derived catalysts: the role of basicity†
Abstract
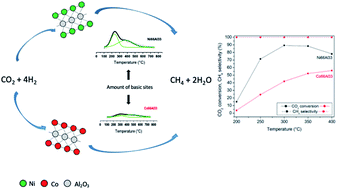
Ni–Al and Co–Al mixed oxides derived from hydrotalcites were prepared by coprecipitation and evaluated in CO2 methanation. The catalysts were characterized by N2 physisorption, XRD, H2-TPR, NH3-TPD, CO2-TPD, TPO and SEM/EDS. The catalytic tests were performed at atmospheric pressure in a fixed-bed reactor, with a gas mixture of CO2/H2/N2 in a ratio of 1/4/15 and GHSV of 60 000 mL gcat−1 h−1, in the temperature range of 200−400 °C. The high density of basic sites and enhanced reducibility presented in the Ni–Al catalyst favored CO2 adsorption, which allows the achievement of around 90% CO2 conversion and 100% CH4 selectivity at 300 °C. The low number of basic sites found in Co–Al was responsible for the lower methanation activity. Both catalysts showed high stability at 400 °C for 5 h without noticeable deactivation, due to the high resistance to sintering associated with the small size of the metal crystallites.


 Please wait while we load your content...
Please wait while we load your content...