Increased hydrogen partial pressure suppresses and reverses hydrogen evolution during Pd catalysed electrolysis of CO2†
Abstract
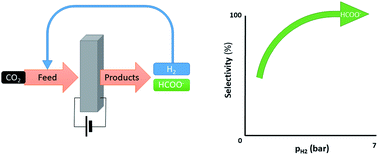
Electrochemical reduction of CO2 on a Pd/C cathode produces formate and hydrogen at low overpotentials. We report on an innovative, effective approach to prevent hydrogen formation. By applying 4 bar partial hydrogen pressure, hydrogen evolution can be fully avoided at −0.05 V vs. RHE and 1 bar partial CO2 pressure.


 Please wait while we load your content...
Please wait while we load your content...