Modeling the response characteristics of photo-sensitive hydrogel electrolytes in Hofmeister salt solution for the development of smart energy storage devices†
Abstract
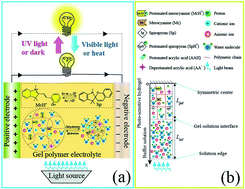
A fascinating feature of photo-sensitive hydrogels with dissolved salts is their light-adjustable ability to conduct electricity. Recently, N-isopropylacrylamide (NIPAAm) polymer electrolytes have been employed to mitigate the thermal runaway of lithium-ion batteries due to the temperature-induced phase transition. Based on this emerging application, we propose an idea in this paper that spirobenzopyran-modified NIPAAm (pSpNIPAAm) hydrogels might be used as smart electrolytes to address the safety issue of renewable energy storage devices through the light-modulated hydration microenvironment. For the design of photo-sensitive hydrogel electrolytes, a multiphysics model is developed to analyze the response characteristic of a pSpNIPAAm hydrogel in saline solution in terms of Hofmeister series. Using this model, the light-to-electrochemical response mechanism is elucidated where the light intensity manipulates the content of each isomer via the photochemical reaction rate, leading to changes in ionic conductivity and electrical potential gradient that are dominated by the hydration state and fixed-charge density within the hydrogel, respectively. After model validation with the help of published experiments, numerical simulation was used to examine the electrochemical and mechanical response of photo-sensitive hydrogels subjected to several external stimuli, such as temperature, ionic strength, and light intensity. It is demonstrated by computational results that the Hofmeister series, charge offset, and buffer pH play an important role in the response characteristic of a hydrogel. The swelling stretch and ionic conductivity collapsed at a temperature that is reduced by 2–5 °C upon changing the salt species from NaCl to Na2SO4 under equivalent light intensity. For photo-stimulated electrical responses, a large charge offset ratio results in significant changes and the buffer pH functions as a polarity switch. These findings provide fundamental cues for the design and optimization of a smart energy storage system based on photo-sensitive hydrogel electrolytes.


 Please wait while we load your content...
Please wait while we load your content...