Highly efficient formic acid and carbon dioxide electro-reduction to alcohols on indium oxide electrodes†
Abstract
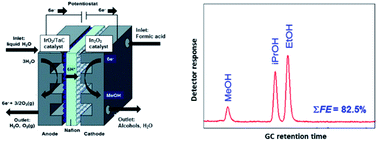
Formic acid is often assumed to be the first intermediate of carbon dioxide reduction to alcohols or hydrocarbons. Here we use co-electrolysis of water and aqueous formic acid in a PEM electrolysis cell with Nafion® as a polymer electrolyte, a standard TaC-supported IrO2 water-splitting catalyst at the anode, and nanosize In2O3 with a small amount of added polytetrafluoroethylene (PTFE) as the cathode. This results in a mixture of methanol, ethanol and iso-propanol with a maximum combined Faraday efficiency of 82.5%. In the absence of diffusion limitation, a current density up to 70 mA cm−2 is reached, and the space-time-yield compares well with results from heterogeneous In2O3 catalysis. Reduction works more efficiently with dissolved CO2 than with formic acid, but the product distribution is different, suggesting that CO2 reduction occurs primarily via a competing pathway that bypasses formic acid as an intermediate.


 Please wait while we load your content...
Please wait while we load your content...