Imidazolium cation enabled reversibility of a hydroquinone derivative for designing aqueous redox electrolytes
Abstract
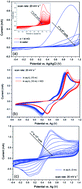
Redox flow batteries are promising devices for large-scale stationary energy storage. One remaining challenge towards their broad application is the rational selection of electrolyte components, composed of redox-active species, and the supporting reaction media. Particularly, good compatibility among the different components should be addressed. Compared to transition metal-based redox electrolytes, green and sustainable organic redox-active materials are becoming favourable due to their low cost, structural diversity and possibly higher volumetric capacity. Herein, we study a hydroquinone species, 2-[(2,5-dihydroxyphenyl)sulfanyl]ethan-1-aminium chloride (SEAHQ), which has high solubility in water, but rather poor chemical and electrochemical stability. Such properties preclude its practical applications. To remove such limitations, we shed light on the unique role of the aqueous supporting electrolytes. Ionic liquids, widely investigated as green reaction media, contain commonly used organic cationic groups and may have better affinity with organic redox materials. This is successfully demonstrated by combining water-soluble imidazolium-based ionic liquids and SEAHQ. Electrochemical characterization experiments showed excellent stability and reversibility of SEAHQ in the studied supporting electrolytes. Redox flow cell tests using SEAHQ as the catholyte with concentrations up to 1 M revealed high cycling efficiencies with current densities up to 100 mA cm−2 over 100 cycles. It is considered that the capacity loss over cycling is related to a continuous decrease in the utilization of the electrolytes, rather than a structural degradation of SEAHQ.


 Please wait while we load your content...
Please wait while we load your content...