CuFe–P from a Prussian blue analogue as an electrocatalyst for efficient full water splitting†
Abstract
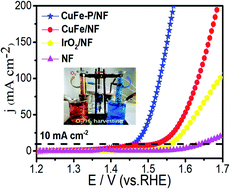
The development of electrocatalysts with abundant reserves and high catalytic activity is a prerequisite for large-scale hydrogen production from electrolyzed water. This article is based on a feasible design idea, using a metal–organic framework (MOF) derivative supported on a foamed nickel (NF) transition metal phosphating catalyst for efficient hydrolysis. In 1.0 M aqueous KOH solution, the CuFe–P/NF electrode only needs an overpotential of 231 mV in the OER to reach 10 mA cm−2, and an overpotential of 153 mV in the HER to reach 10 mA cm−2. It is encouraging that the alkaline electrolytic cell assembled with CuFe–P/NF electrodes can reach a current density of 10 mA cm−2 at an extremely low voltage of 1.577 V, which is currently one of the lower values achieved by non-precious metal electrocatalysts. This work undoubtedly provides a new strategy that can be implemented on a large scale for the synthesis of highly efficient, dual-functional MOF derivative electrocatalysts.


 Please wait while we load your content...
Please wait while we load your content...