Novel Ru nanoparticle catalysts for the catalytic transfer hydrogenation of biomass-derived furanic compounds†
Abstract
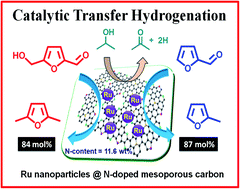
The catalytic transfer hydrogenation (CTH) reaction was investigated for boosting the reduction of biomass-derived furanic compounds to obtain high-quality liquid biofuels. The CTH of 5-hydroxymethylfurfural (HMF) to 2,5-dimethylfuran (DMF) and furfural to 2-methylfuran (MF) was thoroughly studied over the Ru, Pd, Au, Pt, Ni, Rh and Cu metal catalysts supported on nitrogen-doped mesoporous carbons (NMCs) by utilizing 2-propanol as a source of hydrogen. The structural characteristics of the materials were examined by employing various physico-chemical methods, such as XRD, N2 sorption, CHN analysis, XPS, FT-IR spectroscopy, H2-TPR, TEM, CO2-TPD, ICP-OES and Raman spectroscopy. The influence of the N content, basicity of the catalyst, reaction temperature, hydrogen donor, nature of the catalyst support and transition metal was systematically investigated with regard to the substrate conversions and product yields. The correlation between the N content (wt%) of the catalysts and the Ru nanoparticle size (nm) and turnover frequency (h−1) was also investigated. Highly dispersed Ru nanoparticles (1.9 nm) supported on NMC displayed admirable catalytic performance in CTH for the conversion of HMF to DMF and furfural to MF. The catalyst Ru–NMC with a good N content (11.4 wt%) gave 84 and 87 mol% yields of DMF and MF, respectively, with 2-propanol as the source of hydrogen under mild reaction conditions. In addition, this catalyst demonstrated excellent recyclability. The better catalytic activity of the Ru–NMC catalyst in the CTH of HMF and furfural was credited to the small size of the Ru metal nanoparticles (1.9 nm), high N content, superior metal–support interaction and mesoporous framework of the catalyst.


 Please wait while we load your content...
Please wait while we load your content...