An in situ formed LiF protective layer on a Li metal anode with solvent-less cross-linking†
Abstract
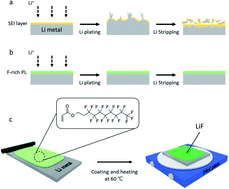
Lithium metal is considered the most promising anode material because of its highest theoretical capacity and lowest electrode potential among the candidate materials. However, Li metal batteries have not been commercialized yet owing to their poor cycle stability. To solve this problem, many studies have reported that the addition of chemically stable LiF to the artificial solid electrolyte interphase (SEI) layer can effectively inhibit the low irreversibility of and dendrite growth on Li metal. However, this measure is inadequate for achieving the desired performance, and it introduces difficulties in the fabrication process. This study employs an in situ LiF protective layer (PL) to stabilize the surface of Li metal. This artificial SEI layer that includes LiF can be fabricated simply through thermal curing of an F-rich material on the surface of Li metal. This “in situ LiF PL” effectively prevents volume expansion of Li metal, resulting in a capacity retention of 52.1% after 1000 cycles at 5.0C. The proposed artificial SEI layer design offers an alternative strategy for stabilizing the surface of Li metal.


 Please wait while we load your content...
Please wait while we load your content...