Boosting photocatalytic H2O2 production by coupling of sulfuric acid and 5-sulfosalicylic acid incorporated polyaniline with g-C3N4†
Abstract
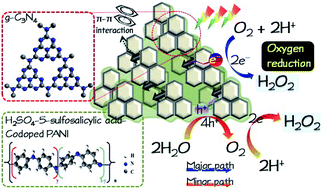
In this study, graphitic carbon nitride (g-C3N4, CN) was decorated with polyaniline (PANI), which was incorporated with inorganic (H2SO4) and organic (5-sulfosalicylic acid, SSA) acids for photocatalytic H2O2 production under simulated solar irradiation. The PANI/CN sample exhibited H2O2 production of approximately 1.5 mM within 3 h, which is much higher than that of either CN or PANI alone in the presence of AgNO3 solution. The increased photocatalytic activity for H2O2 production could be ascribed to the extended visible-light absorption, large specific surface area, and reduced electrical resistance as well as the appropriate redox potential. It should be noted that doping of H2SO4 on PANI could lead to a high oxidation level, which can increase the electrical conductivity of PANI/CN, while the doping of SSA on PANI may induce delocalization and, hence, the enlargement of the specific surface area of PANI. Additionally, CN could afford electron transit through π–π* between the conjugate units of PANI and its tri-s-triazine units, facilitating effective separation for photoexcited electron–hole pairs. This study demonstrated that the doping of PANI with H2SO4 and SSA and coupling with g-C3N4 is a promising technique for photocatalytic H2O2 production utilizing solar energy.


 Please wait while we load your content...
Please wait while we load your content...