The in situ morphology transformation of bismuth-based catalysts for the effective electroreduction of carbon dioxide†
Abstract
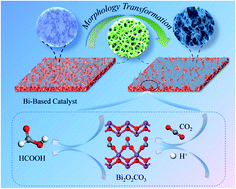
Bismuth (Bi)-based catalysts have been widely used for the electroreduction of carbon dioxide (CO2). In this work, a Bi-based catalyst was electrodeposited on a Cu foam substrate and applied to the selective electrochemical reduction of CO2 to formic acid (HCOOH). An in situ morphological transformation phenomenon accompanied by the formation of petal-shaped bismuth subcarbonate (Bi2O2CO3) nanosheets was observed, and this resulted in enhanced electrocatalytic performance. By using this catalyst, the faradaic efficiency of the CO2 to HCOOH process reached 92% at −1.6 V (vs. Ag/AgCl, −1.0 V vs. RHE) with a total current density of 10 mA cm−2. Also, this electrocatalyst exhibited good stability during electrocatalysis for 20 h. Density functional theory calculations revealed that in situ-formed Bi2O2CO3 species enhanced the catalytic activity by stabilizing the *OOCH intermediate through the stronger orbital hybridization of Bi 6p of Bi2O2CO3 with O 2p of *OOCH. As such, it can be considered that the rate-limiting step in the CO2 electroreduction process should be the second electron transfer step, which is consistent with the Tafel slope analysis results.


 Please wait while we load your content...
Please wait while we load your content...