High-efficiency Ni–P catalysts in amorphous and crystalline states for the hydrogen evolution reaction†
Abstract
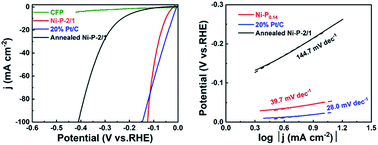
As a kind of catalyst with great development potential, the nickel–phosphorus (Ni–P) catalyst is widely studied. However, the difference in catalytic performance between the amorphous and crystalline Ni–P electrodes in hydrogen evolution still lacks the necessary research. Herein, an amorphous Ni–P electrode was prepared by a method of cathodic electrodeposition, and showed excellent catalytic performance, achieving a HER current density of 10 mA cm−2 in 1.0 M KOH solution at an overpotential of 50 mV. Meanwhile, it was converted to a crystalline Ni–P electrode by annealing under vacuum. On this basis, we have compared the differences in the catalytic performance of the crystalline and amorphous Ni–P electrodes in alkaline and acidic environments. It was found that amorphous Ni–P showed excellent hydrogen evolution catalytic performance in alkaline electrolytes, which was mainly due to the better hydrophilicity and higher inherent catalytic activity of the amorphous state. But it was not stable, so the performance was not satisfactory in acidic solution. On the other hand, the inherent catalytic activity and hydrophilicity of crystalline Ni–P are somewhat worse than those of amorphous Ni–P, and the catalytic performance was not as good as that of the latter, but due to the stable chemical state, crystalline Ni–P had better stability in acidic solutions.


 Please wait while we load your content...
Please wait while we load your content...