Effect of synthesis pH and EDTA on iron hexacyanoferrate for sodium-ion batteries†
Abstract
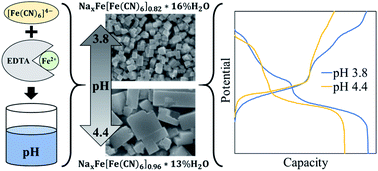
Iron hexacyanoferrate (FeHCF) particles were synthesized at room temperature with ethylenediaminetetraacetic acid (EDTA) at varying pH. The presence of EDTA produced faceted particles and increasing synthesis pH resulted in slower reaction kinetics and larger particles with lower water content and fewer anion vacancies determined by TGA and Mössbauer spectroscopy. Electrochemical testing of sodium metal half cells revealed higher capacity in FeHCF particles grown at lower pH with EDTA, obtaining a maximum discharge capacity of 151 mA h g−1 with 79% capacity retention after 100 cycles at 100 mA g−1 and a rate capability of 122 mA h g−1 at 3.2 A g−1. In contrast, particles grown at higher pH had stunted low-spin Fe redox activity but with improved long-term cyclic stability. These findings demonstrate that small changes in synthesis pH can greatly affect the growth and electrochemical properties of FeHCF when using a pH sensitive chelating agent such as EDTA.


 Please wait while we load your content...
Please wait while we load your content...