Nanoporous NiAl-LDH nanosheet arrays with optimized Ni active sites for efficient electrocatalytic alkaline water splitting†
Abstract
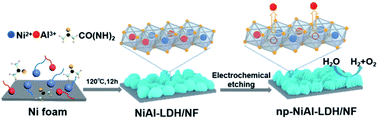
The rational design and development of cost-effective and high-efficiency bifunctional electrocatalysts for overall water splitting is of prime significance for advancing large-scale commercial hydrogen production. Herein, a novel nanoporous NiAl layered double hydroxide nanosheet array with optimized Ni active sites on nickel foam (np-NiAl-LDH/NF) is prepared by a facile and simple metal-defect engineering strategy. We demonstrate that the partial leaking of catalytically inactive Al sites in NiAl-LDH nanosheets during anodic electrochemical etching can not only trigger the generation of a nanoporous structure and electron-rich Ni2+ sites, but can also increase the content of Ni3+ active sites accompanied by abundant oxygen defects, resulting in the synchronously increased density and intrinsic activity of Ni active sites and also enhanced mass and electron transport, thus greatly promoting the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) kinetics. As expected, the as-obtained np-NiAl-LDH/NF electrode needs extremely small overpotentials of 90 and 180 mV to deliver the 10 mA cm−2 current density for the HER and OER in alkaline media, respectively. Moreover, an alkaline electrolyzer assembled with np-NiAl-LDH/NF functioning as both the anode and cathode can yield 10 and 100 mA cm−2 at quite low voltages of ∼1.5 and 1.7 V, respectively, far outperforming the Pt/C/NF–IrO2/NF-coupled electrolyzer, and can give durable stability for at least 60 h. This work provides new insights for creating highly efficient Ni-based electrocatalysts toward overall water splitting via engineering on catalytically active Ni sites.


 Please wait while we load your content...
Please wait while we load your content...