Interplay between halides in the electrolyte and the chemical states of Cu in Cu-based electrodes determines the selectivity of the C2 product†
Abstract
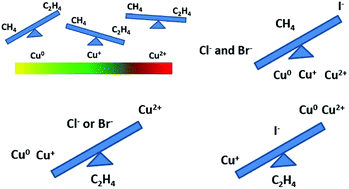
Although still poorly understood, electrolytes can play a key role in obtaining the desired product from the electrochemical reduction of CO2. To address this issue, it is of great significance to better understand the relationship between the selectivity and the activity of the reaction and the electrode structure and the electrolyte. Herein, the influence of electrolytes, especially those that contain halide ions, on the CO2 reduction performance was studied using Cu-based electrodes with various surface components, including Cu0, Cu+ and a mixed state of Cu0, Cu+ and Cu2+. The results show that Cl− and Br− improved the CH4 selectivity for all tested Cu-based electrodes, while the effect of I− was very sensitive to the surface state of Cu. On the one hand, I− could corrode Cu2O and form CuI, which could stabilize Cu+, resulting in a higher faradaic efficiency for C2H4. On the other hand, I− had a negative effect on C2H4 selectivity in the presence of Cu0 and Cu2+. When Cu+ was present on the electrode surface, the ratio of C2H4/CH4 was significantly changed from 19.3 (in KHCO3 electrolyte) to 372.1 (in KHCO3 + KI electrolyte). Our findings improve the understanding of the cooperation between the catalyst and halide electrolytes to produce the desired product and, in particular, the C2 product.


 Please wait while we load your content...
Please wait while we load your content...