Conversion of magnesium waste into a complex magnesium hydride system: Mg(NH2)2–LiH†
Abstract
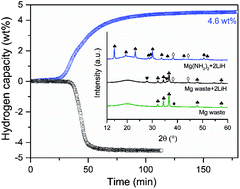
The Mg(NH2)2–2LiH composite has been regarded as a promising on-board hydrogen storage material, due to its favorable thermodynamics and high H2 gravimetric capacity. In this paper, a new route to synthesize the Mg(NH2)2–2LiH composite, starting from hydrogenated Li2Mg(NH)2, is presented. Li2Mg(NH)2 is obtained from the decomposition of a mixture of a magnesium waste alloy (AZ91) and LiH, after a multi-step treatment. The as-prepared Mg(NH2)2–2LiH with Mg coming from AZ91 shows improved hydrogen storage properties, as compared with that synthesized from pure magnesium powder, with a lower dehydrogenation peak temperature of about 15 °C. The reaction pathways during the conversion of the AZ91 alloy to Mg(NH2)2–2LiH are investigated by ex situ and in situ XRD techniques. The obtained results confirm that this new approach is an effective way to recycle magnesium waste alloys into lightweight hydrogen storage materials. In addition, this new technology is easy to scale up and reduces the cost of Mg(NH2)2–2LiH, which has important significance for its practical applications.


 Please wait while we load your content...
Please wait while we load your content...