Immobilized iridium complexes for hydrogen evolution from formic acid dehydrogenation†
Abstract
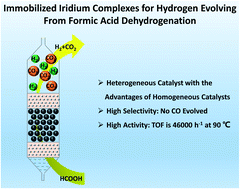
Formic acid dehydrogenation has attracted plenty of attention lately due to its atom-economical method for hydrogen production. Iridium complexes are outstanding homogeneous catalysts which have high activity and selectivity for formic acid dehydrogenation. However, they cannot be well employed in a controllable hydrogen evolution device due to their resolvability. In this research, we report a series of immobilized iridium complexes for formic acid dehydrogenation. Iridium complexes are immobilized by various insoluble N-incorporated polymers, which make the homogeneous catalysts insoluble in most common solvents. We find that the types of N-incorporated group in the polymers will have great influences on the catalytic activity of the immobilized iridium complexes for formic acid dehydrogenation. The morphology of polymers, like specific surface area and particle size, will have influences on the catalytic activities. The turnover frequency (TOF) is up to 46 000 h−1 at 90 °C when we employ Cp*IrCl2(ppy) for formic acid dehydrogenation. We also make a portable fixed bed reactor for hydrogen evolution with the immobilized iridium complexes which could generate gas at 11.2 mL min−1. The immobilized iridium complexes can realize the hydrogen storage, controllable hydrogen production and hydrogen utilization of formic acid under mild conditions.


 Please wait while we load your content...
Please wait while we load your content...