In-depth understanding of the CO2 limitation of air fed anion exchange membrane fuel cells†
Abstract
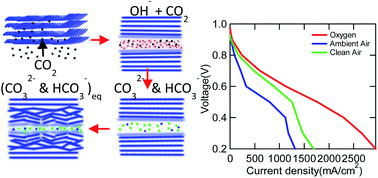
The interaction of a perfluorinated anion exchange membrane (AEM), initially in the hydroxide form, with atmospheric CO2 at 60 °C and under a range of relative humidity conditions is studied both in a fuel cell and with ex situ measurements to understand the performance drop. A new novel titration method was used to quantify the amounts of hydroxide, carbonate and bicarbonate in the membrane. However, hydroxide and bicarbonate react internally which disturbs the equilibrium and hence it's impossible to detect real species concentration using titration. The uptake of CO2 leads to a rise in membrane mass within the first 15 min. The anionic conductivity of the AEM experiences a quick drop within 20 minutes to carbonate and bicarbonate levels. However, switching the inlet gas to 0 ppm CO2 reverses the equilibrium due to the desorption phenomenon. Investigating the morphology of the film by small angle X-ray scattering shows that the ionomer domains lose intensity as the reaction progresses, and the drop is of the double-exponential type but the time of equilibration is slower when compared to that of the conductivity. The wide-angle X-ray scattering data were fit to 3 Gaussian peaks showing that the CF2 inter-chain spacing becomes less crystalline during the process. 30% of peak power was lost for this membrane in an AEM fuel cell on addition of CO2, yet we observed the highest H2/ambient air (400 ppm CO2) performance, 446 mW cm−2, reported to date.


 Please wait while we load your content...
Please wait while we load your content...