An MOF-derived C@NiO@Ni electrocatalyst for N2 conversion to NH3 in alkaline electrolytes†
Abstract
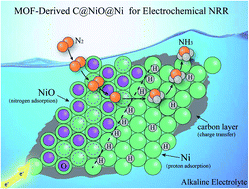
Today, industrial ammonia synthesis mainly depends on the Haber–Bosch process, which causes a lot of energy consumption and huge CO2 emissions. The electrochemical N2 reduction reaction (NRR) is considered a more sustainable and environmentally benign alternative to produce ammonia, but it requires an efficient catalyst to overcome the difficulty of N2 activation. In this work, we reported that MOF-derived C@NiO@Ni microtubes behaved as a high-efficiency electrocatalyst in 0.1 M KOH electrolyte. This electrocatalyst achieved a high NH3 yield of 43.15 μg h−1 mgcat.−1 and faradaic efficiency of 10.9% at −0.7 V vs. a reversible hydrogen electrode. The experimental results indicated that the excellent NRR performance originated from the oxygen vacancies in NiO. Moreover, the abundant NiO/Ni interfaces were conducive to proton adsorption and further enhanced the NRR performance.
- This article is part of the themed collection: Sustainable Energy & Fuels Cover Art


 Please wait while we load your content...
Please wait while we load your content...