Ni loaded on N-doped carbon encapsulated tungsten oxide nanowires as an alkaline-stable electrocatalyst for water reduction†
Abstract
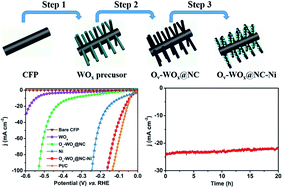
Exploring efficient and economical electrocatalysts for the alkaline hydrogen evolution reaction (HER) is of great importance for the ever-broader applications of hydrogen energy. Tungsten oxides have been long expected to be promising acidic HER electrocatalysts, whereas the poor intrinsic stability in an alkaline electrolyte and inferior electrical conductivity hamper their application in the alkaline HER. Herein, we design a hierarchical electrocatalyst, Ni loaded on N-doped carbon shell coated oxygen-vacancy-rich WOx nanowires. The composite shows an overpotential of 67 mV at 20 mA cm−2 and 164 mV at 100 mA cm−2 in 1 M KOH, which is highly comparable to that of the commercial Pt/C catalyst. It also shows long-term electrochemical durability over 20 h. The excellent HER performance of the electrocatalyst is attributed to the synergetic catalytic effects between the highly dispersed Ni nanoparticles and the N-doped carbon-encapsulated WOx nanowires with rich oxygen vacancies, as well as the carbon shell endowing enhanced chemical stability.


 Please wait while we load your content...
Please wait while we load your content...