An iron foam acts as a substrate and iron source for the in situ construction of a robust transition metal phytate electrocatalyst for overall water splitting†
Abstract
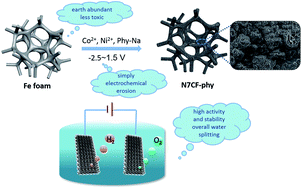
A metal iron foam benefiting from a three-dimensional skeleton and providing abundant active metal sites is an optimal alternative option for directly developing an economical and highly efficient water splitting electrocatalyst, but it is seldom reported. Herein, we adopted a one-step electrochemical erosion method to oxidize the iron foam surface and then, iron ions in situ chelated with bi-metallic phytates to afford stereo tri-metallic compounds. The introduction of the bimetal and phytates enabled the electrode to create more catalytic sites and construct a more complicated tridimensional structure, which enlarged the active surface area and improved the water splitting performance. Consequently, the as-prepared N7CF-phy electrode delivered higher current densities at lower overpotentials for the oxygen and hydrogen evolution reactions in 1 M KOH. After being assembled as an electrolyzer for overall water splitting, the electrodes still exerted a satisfying performance. Moreover, the sustained alternating chronopotentiometric measurements for N7CF-phy as both the oxygen and hydrogen evolution reaction (OER and HER) catalysts clarified the outstanding validity and robust durability.


 Please wait while we load your content...
Please wait while we load your content...