Synthesis and hydrogen evolving catalysis of a panchromatic photochemical molecular device†
Abstract
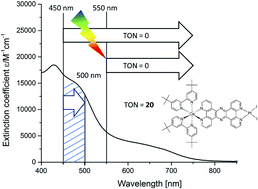
A dinuclear hydrogen evolution photocatalyst [(tbbpy)2Os(tpphz)PtI2](PF6)2 (tbbpy = 4,4′-tert-butyl-2,2′-bipyridine; tpphz = tetrapyrido[3,2-a:2′,3′-c:2′′,3′′-h:2′′′,3′′′-j]phenazine) is synthesized in order to make use of the broader range of visible light absorption mitigated by the osmium center. In a first step, the activity of the complex for hydrogen evolution is investigated by evaluating the role of different electron donors (triethylamine (TEA), 1-benzyl-1,4-dihydronicotinamide (BNAH) and 1,3-dimethyl-2-phenyl-2,3-dihydro-1H-benzo[d]-imidazole (BIH)). The highest photocatalytic activity (TON(H2) of 59) was observed for BIH as the electron donor. UV-vis investigations during catalysis with 470 nm (LED) irradiation show the rise of an absorption band at around 600 nm during catalysis, which indicates the reduction of the bridging ligand. Interestingly, broadband light excitation with wavelengths >450 nm induces no catalytic behavior. An intra-ligand charge transfer transition within the reduced tpphz moiety is hypothesized in order to rationalize the breakdown of the catalysis by broad band excitation.
- This article is part of the themed collection: 3rd International Solar Fuels Conference and International Conference on Artificial Photosynthesis


 Please wait while we load your content...
Please wait while we load your content...