Intramolecular Friedel–Crafts alkylation with a silylium-ion-activated cyclopropyl group: formation of tricyclic ring systems from benzyl-substituted vinylcyclopropanes and hydrosilanes†
Abstract
A trityl-cation-initiated annulation of benzyl-substituted vinylcyclopropanes (VCPs) with hydrosilanes is reported. Two Si–C(sp3) bonds and one C(sp2)–C(sp3) bond are formed in this process where an intramolecular 6-endo-tet Friedel–Crafts alkylation of a silylium-ion-activated cyclopropane ring is the rate-determining key step. The reaction mechanism is proposed based on computations and is in agreement with experimental observations. The new reaction leads to an unprecedented silicon-containing 6/6/5-fused ring system. A phenethyl-substituted VCP derivative yields another unknown tricycle having 6/6/6 ring fusion by reacting in a related but different way involving a 6-exo-tet ring closure.
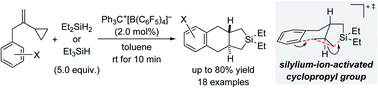
- This article is part of the themed collection: 2020 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...