Aromatic foldamers as scaffolds for metal second coordination sphere design†
Abstract
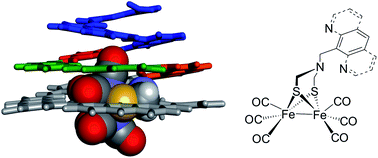
As metalloproteins exemplify, the chemical and physical properties of metal centers depend not only on their first but also on their second coordination sphere. Installing arrays of functional groups around the first coordination sphere of synthetic metal complexes is thus highly desirable, but it remains a challenging objective. Here we introduce a novel approach to produce tailored second coordination spheres. We used bioinspired artificial architectures based on aromatic oligoamide foldamers to construct a rigid, modular and well-defined environment around a metal complex. Specifically, aza-aromatic monomers having a tethered [2Fe–2S] cluster have been synthesized and incorporated in conical helical foldamer sequences. Exploiting the modularity and predictability of aromatic oligoamide structures allowed for the straightforward design of a conical architecture able to sequester the metal complex in a confined environment. Even though no direct metal complex–foldamer interactions were purposely designed in this first generation model, crystallography, NMR and IR spectroscopy concurred to show that the aromatic oligoamide backbone alters the structure and fluxional processes of the metal cluster.


 Please wait while we load your content...
Please wait while we load your content...