Iron-catalyzed α-C–H functionalization of π-bonds: cross-dehydrogenative coupling and mechanistic insights†
Abstract
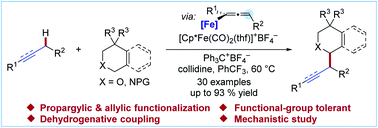
The deprotonation of propargylic C–H bonds for subsequent functionalization typically requires stoichiometric metal alkyl or amide reagents. In addition to the undesirable generation of stoichiometric metallic waste, these conditions limit the functional group compatibility and versatility of this functionalization strategy and often result in regioisomeric mixtures. In this article, we report the use of dicarbonyl cyclopentadienyliron(II) complexes for the generation of propargylic anion equivalents toward the direct electrophilic functionalization of propargylic C–H bonds under mild, catalytic conditions. This technology was applied to the direct conversion of C–H bonds to C–C bonds for the synthesis of several functionalized scaffolds through a one-pot cross dehydrogenative coupling reaction with tetrahydroisoquinoline and related privileged heterocyclic scaffolds. A series of NMR studies and deuterium-labelling experiments indicated that the deprotonation of the propargylic C–H bond was the rate-determining step when a Cp*Fe(CO)2-based catalyst system was employed.
- This article is part of the themed collection: 2020 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...